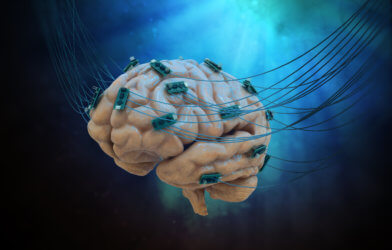
German drugmaker, Merck KGaA, and biotech startup, neuroloop, have joined forces in efforts to create a novel treatment for inflammatory diseases. Using a neurostimulator device, used by neuroloop to lower blood pressure among other applications, Merck KGaA seeks to treat chronic inflammatory conditions such as arthritis and IBS.
Belén Garijo, chief executive of Merck, says that the neurostimulator device “shows great promise in helping to improve therapeutic outcomes and efficiency for patients with chronic inflammatory diseases.”
The collaboration unites neuroloop’s expertise in neurostimulation with Merck’s experience in electronics, medicines and drug delivery.
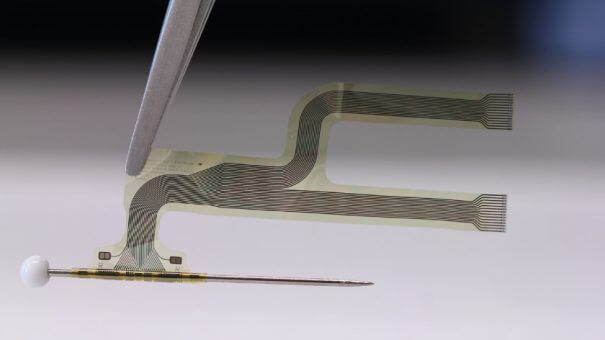
neuroloop is an early-stage healthcare spin-off from Freiburg University with investments from B. Braun Melsungen. They have developed a cuff electrode that delivers selective stimulation of vagus nerve fibres in order to carry signals from the body to the brain and back again. One of these neural circuits has been named the ‘inflammatory reflex’ and is thought to be responsible for the delivery of inflammation information to the brain and anti-inflammatory instructions to the affected tissues.
Michael Lauk, chief executive of neuroloop, cautions that “identifying the specific disease relevant nerve signal patterns and subsequently modulating these signals via stimulation are major challenges in the field of bioelectronics.”
“Together with the strong preclinical and clinical expertise of Merck and our platform which enables multi-channel selective stimulation, we are well positioned to potentially solve these crucial challenges and offer neurostimulator treatment to patients suffering from chronic inflammatory diseases,” he added.
A promising study of the vagus nerve and reactions to a stimulating implant was published in 2016 which showed that the device was successful in reducing the production of inflammatory proteins in patients with rheumatoid arthritis.
The players in this research hope to have initial trial data by the end of 2022 so they can then create a clinical development plan for the bioelectronic device with hopes to get approval in European and US markets.
Merck has made bioelectronics their primary research focus and claims that success with bioelectronic devices could mean the ability for healthcare providers to monitor disease conditions and progression in patients.






-392x250.jpg)

Comments