Parkinson’s disease is common, but despite its prevalence and voluminous research attempting to determine its pathophysiology, it remains an enigma. Recently, however, the diligence of scientists at the Scripps Research Institute in La Jolla, California has been rewarded with new and significant findings regarding the progression of disease. Their discovery may lead to interventions which could dramatically slow, or even stop, progression of the disease.
The recently-released article reports that Parkinson’s disease may be driven, in part, by stress-related biochemical events that interfere with an essential cellular cleanup system. The system disturbance allows the spread of harmful protein aggregates in the brain.
“We think our findings about this apparent disease-driving process are important for developing compounds that can specifically inhibit the process of disease spread in the brain,” says Dr. Stuart Lipton, a professor in the Department of Molecular Medicine at TSIR, in a statement.
Parkinson’s disease affects roughly one million people in the United States. It is the second most common neurodegenerative disease (Alzheimer’s disease is first.) The inciting condition is unknown, but it involves the deaths of neurons in a characteristic sequence in key brain regions. The death of dopamine-producing neurons in the midbrain leads to the signature Parkinsonian tremor and other movement impairments. Damage to different brain regions results in other disease signs, including dementia in advanced stages of PD. It is often associated with another disease, Lewy body dementia (LBD).
Both diseases have affected neurons which contain abnormal protein aggregates known as Lewy bodies, composed predominantly of alpha-synuclein protein. Previously, it has been shown that alpha-synuclein protein aggregates spread from neuron to neuron in both Parkinson’s and LBD, apparently transmitting the disease process through the brain. How alpha-synuclein aggregates collect and spread has been unclear.
A clue previously uncovered by Lipton’s lab and others is that the Parkinson’s and LBD disease processes produce highly reactive nitric oxide. These reactive nitrogen molecules disrupt important cellular systems, including systems that usually keep protein aggregates under control.
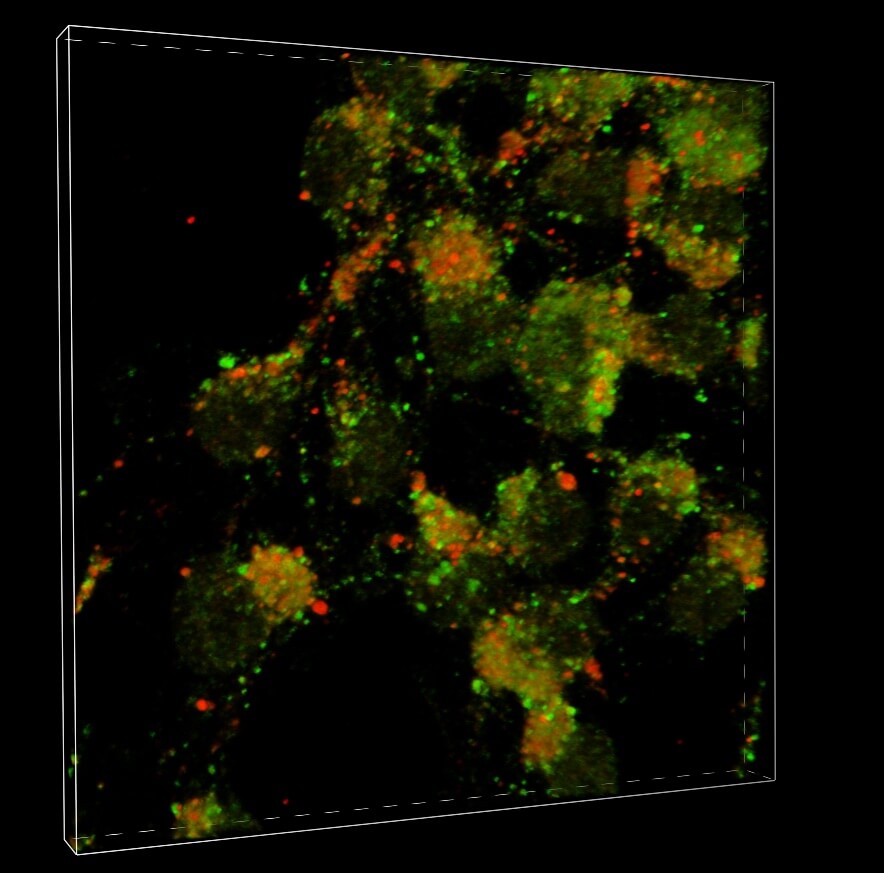
The new study validates that mechanism by showing that a type of nitrogen-molecule reaction called S-nitrosylation affects an important cellular protein called p62. The process triggers the collection and spread of alpha-synuclein aggregates.
The p62 protein assists a waste-management system that helps cells eliminate harmful protein aggregates. The researchers found evidence that in cell and animal models of Parkinson’s, p62 is S-nitrosylated at abnormally high levels of affected neurons. This alteration causes a buildup of alpha-synuclein aggregates. It leads to the secretion of the aggregates by affected neurons, which are then taken up by nearby neurons.
“The process we observed seems very similar to what is seen in Parkinson’s and LBD brains,” says study first author Chang-Ki Oh, a staff scientist in the Lipton laboratory.
Lipton and Oh say that S-nitrosylation of proteins increases in cellular stress, including the presence of protein aggregates. Thus, this modification of p62 could be key in a self-reinforcing process that stresses brain cells beyond their limits, while spreading the source of stress to other brain cells.
The team is developing drugs that specifically inhibit the S-nitrosylation of p62, but it may take years to develop commercial drugs that slow the progression and prevent further spread of Parkinson’s disease and LBD.





-392x250.jpg)