A recent study by researchers at Weill Cornell Medicine has found a way to employ new imaging techniques to quickly and accurately identify the functions of a group of light-sensing molecules. In the emerging field of optogenetics, a tool which controls neurons and other cells using light pulses, these findings may be an important step toward the development of new strategies for use in research and beyond.
Light-sensitive proteins are critical to processes like photosynthesis and vision, making studying their functions of key significance for many researchers. For further understanding of light-sensitive proteins, scientists typically look to research on a protein known as bacteriorhodopsin. In many single-celled organisms, bacteriorhodopsin is the protein responsible for the process of photosynthesis.
In previous studies of bacteriorhodopsin, researchers have pieced together its three-dimensional structure and observed its functions. However, there is much still unknown about the protein due to gaps in models created by limited current techniques.
‘Current challenge is to assess kinetics’
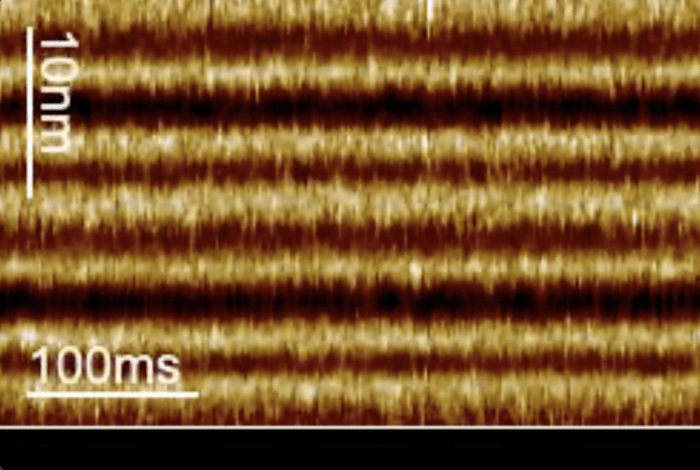
Researchers at Weill Cornell Medicine set out to develop a new technique that would address these limitations. Their technique, known as line-scanning high-speed atomic force microscopy, works by capturing light responsive movements of bacteriorhodopsin on a millisecond time scale.
“The solution of protein structures has become quite straightforward, but a current challenge is to assess kinetics, which provide a dynamic understanding of the system,” says senior author Dr. Simon Scheuring, professor of physiology and biophysics in anesthesiology at Weill Cornell Medicine, in a statement.
A particular struggle, researchers said, was to find a way to capture protein changes over short time spans, as current methods tracking individual molecule activity work too slowly to do so. Using these techniques to attempt to monitor quick shape changes of proteins — like bacteriorhodopsin in response to light — would be akin to taking a video with the camera set to a slow shutter speed, remarked Dr. Scheuring. While you may see a fast moving bird on one side of the frame and again at the other, you will miss all that happened in between.
Previously, researchers used weaker versions of the protein to observe its movements.
“Up to now, to study the kinetics of bacteriorhodopsin, people were using mutants that were slower,” says lead author Dr. Alma Perez Perrino, a postdoctoral fellow in Dr. Scheuring’s laboratory.
But, these slower mutant proteins were not representative of normal activity. In their search for a better way to understand bacteriorhodopsin, Dr. Perez Perrino worked with a team of colleagues to develop a technique called line-scanning high-speed atomic force microscopy. Rather than capturing a quality image further apart and losing activity in the middle, this method collects lower quality images in a shorter timespan. Or, as in Dr. Scheuring’s example, Dr. Perez Perrino’s technique would be like taking multiple blurry images of the bird all the way across the screen.
“We are tracking the protein every 1.6 milliseconds, so we could explore the speed of the wild-type bacteriorhodopsin,” notes Perez Perrino.
‘You want a switch on a process’
Bacteriorhodopsin alternates between an open and closed state in response to light. With the use of faster imaging techniques available, researchers were able to observe the states under differing conditions. Their observations found that while the transition to and duration of the open state remains consistent at the same speed, the molecule stays in the closed state for a longer duration while light intensity decreases.
Researchers in the field of optogenetics have enabled light-sensing molecules in neurons or other cells to change cell behavior with light pulses by inserting additional genes. Future potential for these findings includes treatment advances for patients with neurological diseases and developments in the study of optogenetics.
“Ultimately, you want to switch on a process, then get the maximum out of it, and be able to switch it off again immediately,” said Dr. Scheuring. “So, it is very important to know the kinetics of the molecules for that switching.”
This study is published in Nature Communications.
Article by Anna Landry