The well-known ADHD medication atomoxetine, better known as Strattera, may be able to slow the effects of neurodegeneration in people with early signs of Alzheimer’s disease. Researchers at Emory Brain Health Center hope their finding could lead to a promising treatment for people suffering from the incurable brain disease.
The results are promising as one of the very first published clinical studies to demonstrate notable effects on the protein Tau, which forms neurofibrillary tangles in the brain of Alzheimer’s disease patients. Six months of atomoxetine treatments normalized several markers of neuro-inflammation and showed reduced levels of Tau protein in the cerebrospinal fluid (CSF) of 39 research participants with mild cognitive impairment (MCI).
Why Strattera is suitable for Alzheimer’s patients
Science has searched for an alternative method to treating Alzheimer’s disease with medication that does not rely on antibodies against Tau or beta-amyloid, another Alzheimer’s related protein. While another FDA-approved drug, aducanumab, has been explored, its benefits are not well-agreed upon by experts.
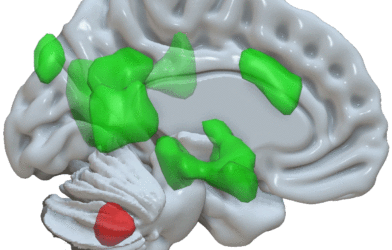
Researchers chose to explore atomoxetine, which is sold commercially as Strattera, as a potential treatment option with the goal of boosting norepinephrine levels in the brain. Norepinephrine is produced mainly by the locus coeruleus, which is the region of the brainstem that seems to first show Alzheimer’s related pathology.
Previous research in rat and mouse models of Alzheimer’s has shown that norepinephrine has positive effects on cognition and pathology. With this in mind, Emory researchers hoped that norepinephrine may be able to stabilize a vulnerable region of the brain against the neurodegeneration associated with Alzheimer’s disease.
“One of the major advantages of atomoxetine is that it is already FDA-approved and known to be safe,” says senior author David Weinshenker, PhD, professor of human genetics at Emory University School of Medicine, in a statement. “The beneficial effects of atomoxetine on both brain network activity and CSF markers of inflammation warrant optimism.”
More atomoxetine research needed
Despite expectation due to the short duration of the study, atomoxetine did not have a significant effect on cognition or other clinical outcomes. While more studies will be required before formal approval can be achieved, the researchers at Emory are optimistic that atomoxetine may be a step forward in the treatment of Alzheimer’s disease.
“We are encouraged by the results of the trial. The treatment was safe, well tolerated in individuals with mild cognitive impairment, and modulated the brain neurotransmitter norepinephrine just as we hypothesized,” says lead author Dr. Allan Levey, a professor of neurology at Emory University School of Medicine and director of the Goizueta Institute at Emory Brain Health. “Moreover, our exploratory studies show promising results on imaging and spinal fluid biomarkers which need to be followed up in larger studies with longer periods of treatment.”
Results from this study leave room for further research. Modern science has made it possible to use MRI techniques to visualize the integrity of the locus coeruleus of a living person, which researchers believe is an important next step in follow-up studies.
The study is published in the journal Brain.
Article written by Anna Landry