The FDA recently announced Investigational New Drug clearance for a novel ketamine treatment for mental health disorders, particularly depression. atai Life Sciences N.V., a clinical-stage biopharmaceutical company aiming to transform the treatment of mental health disorders, now has clearance to conduct a clinical DDI study of PCN-101 (R-ketamine). atai plans to initiate the study early this year through its platform company Perception Neuroscience.
According to the World Health Organization (WHO), an estimated 280 million people live with depression globally. About one-third of this population is undertreated or experiences treatment-resistant depression (TDR). “TRD represents a large percentage of people with severe, difficult to treat depression who have failed to sufficiently respond to at least two different antidepressant treatments,” explains Terence Kelly, PhD, CEO of Perception Neuroscience in a statement. “We believe that PCN-101 has the potential to offer a differentiated therapeutic effect, in terms of both efficacy and ease of administration, for clinicians and patients, as a potentially rapid-acting antidepressant. We look forward to progressing its clinical development.”
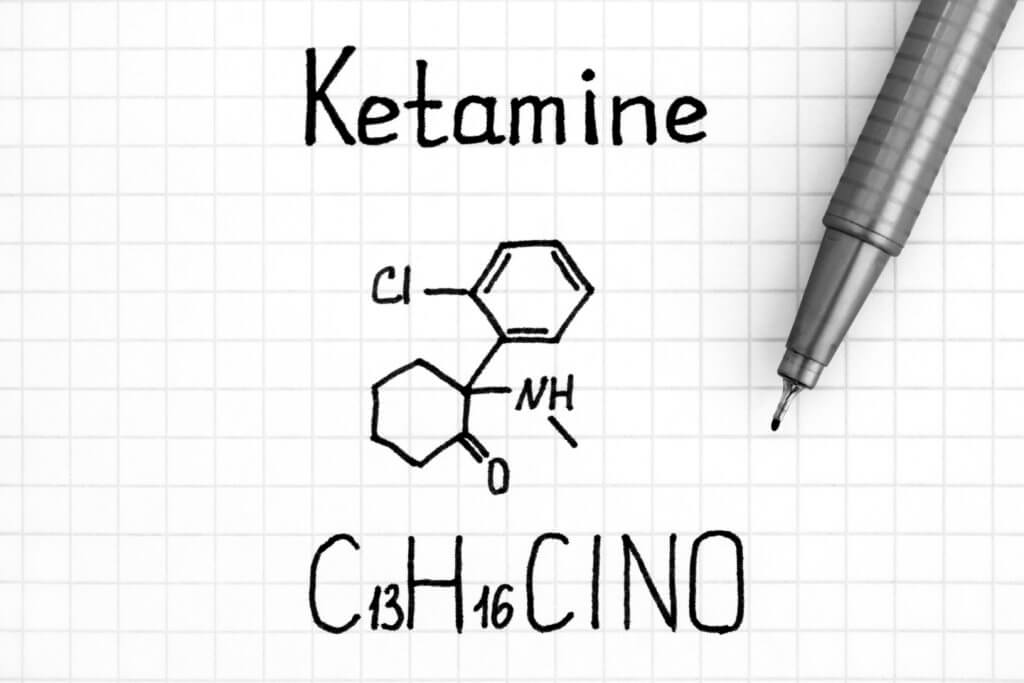
Ketamine is a dissociative anesthetic. Doctors use it to induce general anesthesia for medical procedures that do not require muscle relaxation. Ketamine can also produce hallucinations similarly to other drugs such as LSD. Ketamine consists of two equal compounds, S-Ketamine and R-Ketamine (PCN-101).
In preclinical animal models of depressive behavior, R-ketamine has demonstrated the potential to offer longer durability and a potentially more favorable tolerability profile than S-ketamine, which could enable the potential for at-home use. In addition, a third-party, open-label study observed a rapid, durable antidepressant response and limited dissociative side effects in patients with TRD after a single intravenous dose of another formulation of R-ketamine.
The unique properties of PCN-101 could offer a differentiated profile to currently available antidepressants and address key patient needs, including the potential of rapid action and anti-suicidal effect. Rapid onset of action is particularly important in this patient population. However, frontline selective serotonin reuptake inhibitors (SSRIs) can take up to 12 weeks before providing maximal benefit, while suicidal ideation affects as much as 30% of TRD patients at least once during their lifetime.
“We see great promise in PCN-101 as a potentially rapid-acting antidepressant with a more favorable safety and tolerability profile than S-ketamine, which could enable at-home use,” said Florian Brand, CEO and Co-Founder of atai Life Sciences. “With today’s IND clearance, we are excited to continue assessing the therapeutic potential of PCN-101 in the U.S., where, like elsewhere in the world, many patients struggle with treatment-resistant depression and desperately need innovative therapeutic options.”
This clinical DDI trial will advance alongside an existing Phase 2a proof-of-concept trial in TRD, recently initiated in Europe. Additionally, atai anticipates running a bioavailability study in 2022, which is designed to bridge the IV formulation to a subcutaneous formulation of PCN-101, supporting the potential for self-administration.







-392x250.jpg)